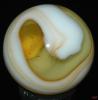
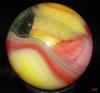
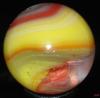
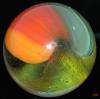
Those examples, that don't look classically like MF or Akro oxblood sure are a puzzle to me (not the one above, btw). I've got some too from other manufacturers that might be oxblood, but fall into that nether-world of 'maybe ox or maybe not.' I have Masters that would be close but I can't tell, but they wouldn't be a classic match to the oxbloods either, and I think here is the challenge. Ox or not (and not referring to other kinds of red either...I'm speaking of that dark, optically flat, red color only).
My understanding is that the formulations for oxblood are very touchy but essentially revolve around a re-dox reaction between copper and iron atoms. This is a complex (for glass making) chemical oxidation-reduction reaction, but I currently remain unclear what is going on, as different readings from different sources are conflicting (I've red multiple accounts and interpretations)...I'm not, at this time, sure which is the current accurate thinking on this. What is clear, is that this is a very touchy reaction: depending on heat, other additives of the glass, presence or absence of levels of oxygen and the length of time the glass is in flux, production of oxblood is a very touchy thing indeed. If stopped at the wrong time in the melt, or the components of the glass, or who know what else, the glass may not turn out classically oxblood.
So, I think the question has always been for me...is oxblood an actual appearance of the glass or the intent of the glass maker? On those 'fence sitting' examples: Was this intended and it just didn't entirely make it to the ox state? Or it only appears to be oxblood. I've always taken MF and Akro to be, classically, the best of oxblood makers (in the manufacturing age). But we've all seen those, hmmm, "is this oxblood?" kinds of examples too...when they clearly don't show the banding and dark ribbons and red striations of coagulated looking blood. I really don't know what to think. What is clear to me is that a glass maker just doesn't simple say, hmmm, "let's make some ox today!," without considering the fickle nature of this stuff; and no doubt, the cost of trying to make it, and then maybe fail, was always a consideration, especially later on. From my reading, this can be very fussy stuff indeed. (incidentally, I've read that ox can get more copper green when the cook/formulation doesn't work right...and I have a really really beat up, probably MF that has ox and tonnes of copper green glass regions and less ox...totally weird and in very very very bad condition...).
Sorry about the on and on...I like chemistry! John